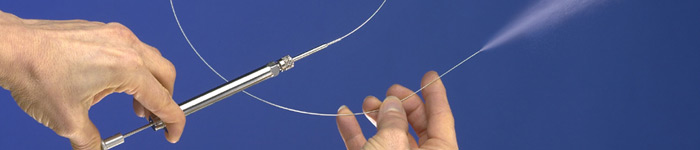
Pioneering more precise, relevant pre-clinical models of pulmonary drug delivery
Pulmonary researchers all over the world have come to rely on Penn-Century’s unique, hand-held devices as the “gold standard” for pre-clinical, in vivo and in vitro models of aerosol delivery. Read our story in Inhalation Magazine.
As the exclusive source of the MicroSprayer® Aerosolizer and the Dry Powder Insufflator™, Penn-Century has made possible the discovery, development and commercialization of hundreds of new pharmaceutical and biologic therapies for pulmonary and systemic diseases.
Our patented intratracheal aerosol technology has been adopted by more than 1,000 organizations in 40 countries. Our customer list includes the world’s leading pharmaceutical and biotech companies, contract research organizations, academic and medical research labs and government and defense institutes.
We put the power of innovation in your hands
Our hand-operated devices have given researchers from global companies to start-ups to academic labs access to a kind of sophisticated and innovative research that used to be available only to organizations with huge research budgets.
We have made pre-clinical pulmonary drug research more precise and more accessible to those whose only alternative previously was to instill large liquid droplets into the lungs with a pipette or catheter, or invest in costly animal nebulizer systems that many researchers report to be slow, imprecise and wasteful. This technology enables researchers to pursue the precise pharmacokinetic/pharmacodynamic data that is so critical to the success of preclinical respiratory research.
More than 1,200 published scientific studies and drug patents cite use of Penn-Century devices. Together they provide compelling evidence for the efficacy of pulmonary and respiratory therapies across a range of diseases and drugs. They also provide dramatic proof of the significant potential for adopting Penn-Century’s unique technology for human clinical use.
Meeting the need for better treatment models of pulmonary and respiratory disease
Penn-Century was launched by founder Ted Century at a time of growing recognition that the lungs offer an extraordinary, but underutilized avenue for treating pulmonary as well as systemic diseases.
Penn-Century evolved to meet the growing demand for more precise, effective, relevant pre-clinical models of pulmonary and respiratory drug delivery. The company was the first to introduce a completely airfree technology for directing precise, measurable doses of liquid aerosol to the lungs and other inaccessible locations. At the time, this was considered “impossible” by leading experts in aerosol science. This discovery was soon followed by patented innovations for precise intratracheal dosing of dry powders.
Today, Penn-Century remains the sole manufacturer of our unique, patented liquid and dry powder intratracheal aerosol devices and accessories. The MicroSprayer® Aerosolizer remains the only airfree method of liquid intratracheal drug delivery. The Dry Powder Insufflator™ remains the only commercially available technology for precise, intratracheal dosing of dry powders.
Our devices offer precision and quantifiability with zero waste, when compared to costly, air-driven animal nebulizer systems, and vastly improved distribution, dose range and safety when compared with liquid bolus instillation via a catheter.
Respiratory drug delivery – a rapidly growing, billion dollar market
Because the lungs offer such broad exposure and rapid access to the body’s vascular system, they offer an attractive route for treating respiratory as well as systemic diseases. Drugs delivered by the pulmonary route have the potential to avoid the metabolic and systemic side effects associated with oral or intravenous drug delivery and the discomfort of drug delivery by injection.
Pulmonary strategies for vaccinations or treatments of a host of chronic, acute and genetic diseases
As research has advanced the scientists have begun to explore whether the lungs could be the preferred avenue to treat not only asthma and emphysema, but other diseases infectious diseases, lung cancer, pulmonary hypertension, tuberculosis, cystic fibrosis, diabetes, bone loss.
In the past 20 years, the growing understanding of the potential for targeting the lungs has fueled a giant leap in investment in respiratory research and development of drug delivery technologies. In 2011, the global pulmonary drug delivery technologies market was expected to reach $22.5 billion.
New methods of respiratory drug delivery – “interventional bronchoscopy”
In recent years, greater investment has also gone into strategies for what has come to referred to as “interventional bronchoscopy.” Because Penn-Century’s liquid and dry powder devices can be custom-made to any length, and inserted in larger animal models via an endotracheal tube or bronchoscope, they have been uniquely suited for researchers wanting to target the carina, the lungs or an individual lesion or location within one lung.
Meeting an unmet need for more precise models of aerosol drug delivery to the lungs
We have made pulmonary drug research more precise and more accessible to those whose only alternative previously was to squeeze liquid droplets into the animal’s lungs with a pipette or invest in costly animal nebulizer systems that our customers tell us are slow, imprecise and wasteful.
At the time Penn-Century’s unique intratracheal aerosol technology was introduced, the choices for in vivo and in vitro models of pulmonary drug delivery were limited to:
- Large, costly, air-driven animal nebulizer systems that blow drugs at the animal’s nose to mimic “inhalation”
- Exposure systems that trap aerosols in an enclosure with a conscious animal or in vitro cell model
- Liquid bolus or droplet instillation in which large drops are dripped into the lung via a pipette or catheter.
These existing methods created significant challenges to researchers
- Large animal nebulizer systems typically dilute the drug with compressed air to render it small enough to pass through the nose of small research animals. Because the aerosol particles are moving at high moment momentum, much of the dose is forced against the nasal passages, creating waste of sample materials. The concentration of drug in the lungs is often inadequate and the method is slow. Most significantly, the method makes it far more difficult to determine the delivered dose, making it more challenging to derive the precise pharmacokinetic/pharmacodynamic data that is so critical to the success of preclinical respiratory research.
- mimic human inhalation in rodent model – given that rodents are nose-only breathers
- draw conclusions about drug safety and efficacy based on achieve high enough concentration to optimize formulation
- draw conclusions about drug safety and efficacy using delivery methods that yield minimal concentration and make the delivered dose very time-consuming and difficult to quantify
- achieve broad, even distribution in the lung, down to the alveoli
Moving beyond the anatomical and technological barriers to innovation
Company founder, Ted Century, solved several major challenges that had previously eluded medical device designers and clinicians: 1) how to safely deliver significant amounts of drugs directly to the lungs in an aerosol form, rather than as a stream of liquid droplets and 2) how to create an aerosol at the end of a long thin tube without reliance on propellants, compressed air, heat, electricity or cumbersome ultrasonic devices. The devices he developed have successfully moved pulmonary research beyond the anatomical and technological barriers that prevented administration of liquid aerosols and dry powders in the past.
The time and labor involved in fabricating the final device is considerable. As with all of our devices, what looks simple and easy to the end user has required a lot of effort on our part. As with all Penn-Century devices, each one is handmade precisely to specification.
